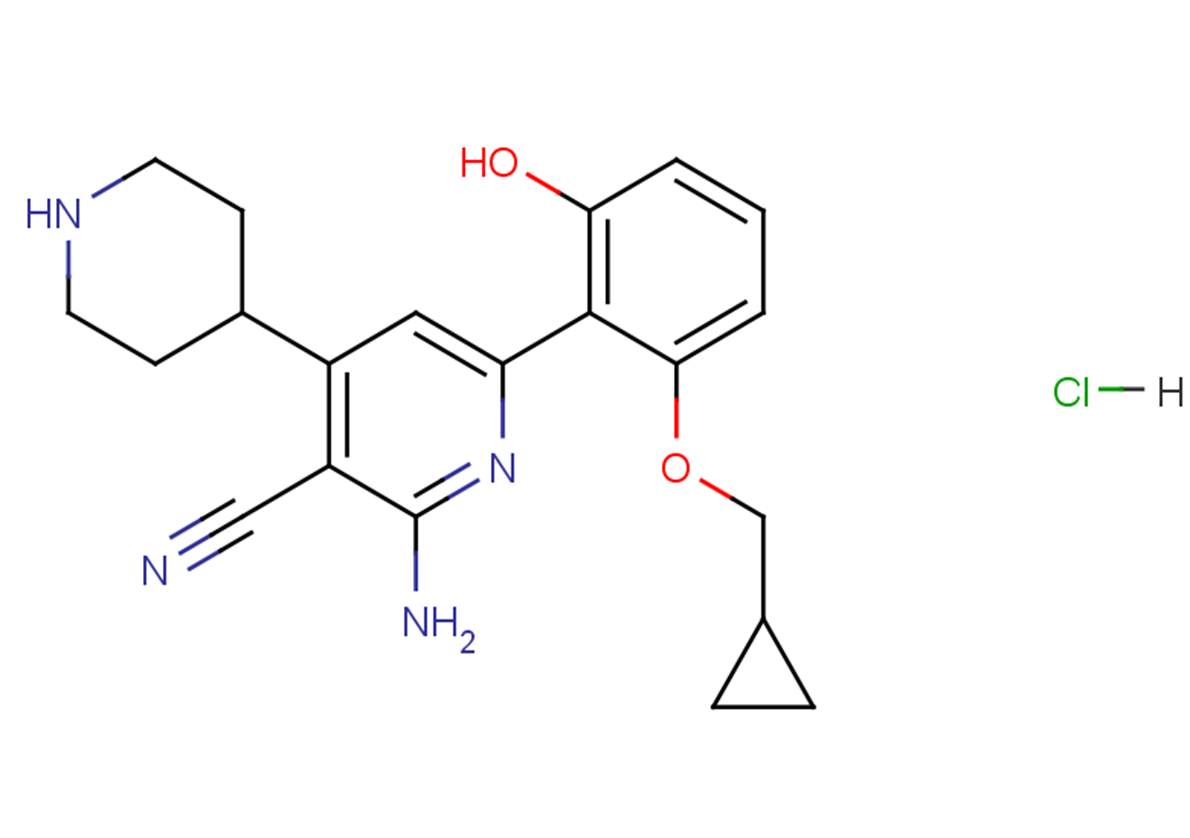
ACHP Hydrochloride
CAS No. 406209-26-5
ACHP Hydrochloride ( IKK-2 Inhibitor VIII )
产品货号. M24367 CAS No. 406209-26-5
ACHP Hydrochronide (IKK-2 Inhibitor VIII) 是一种高效、选择性 IKK-β 抑制剂 (IC50: 8.5 nM)。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 5MG | ¥1450 | 有现货 |


|
| 10MG | ¥2373 | 有现货 |


|
| 25MG | ¥3953 | 有现货 |


|
| 50MG | ¥5630 | 有现货 |


|
| 100MG | ¥8060 | 有现货 |


|
| 500MG | ¥16119 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称ACHP Hydrochloride
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述ACHP Hydrochronide (IKK-2 Inhibitor VIII) 是一种高效、选择性 IKK-β 抑制剂 (IC50: 8.5 nM)。
-
产品描述ACHP Hydrochloride (IKK-2 Inhibitor VIII) is a highly potent and selective IKK-β inhibitor (IC50: 8.5 nM).
-
体外实验ACHP Hydrochloride (Compound 4j) exhibits potent IKK-β inhibitory (IC50: 8.5 nM) and cellular activities (IC50=40 nM, in A549 cells). ACHP moderately inhibits IKK-α with an IC50 of 250 nM but exhibits good selectivity towards other kinases, such as IKK3, Syk and MKK4 (IC50>20,000 nM). Moreover, ACHP demonstrates quite potent activity in various cellular assays. ACHP inhibits NF-κB-dependent reporter gene activation in TNFα-activated HEK293 cells and PMA/calcium ionophore-activated Jurkat T cells. ACHP fails to inhibit PMA-induced AP-1 activation in MRC-5 cells and PMA/calcium ionophore induced NF-κB dependent reporter gene transcription in Jurkat cells even at concentrations exceeding 10 μM. ACHP selectively interferes with the NF-κB signaling cascade by inhibition of IKK-β in living cells. ACHP inhibits the growth of these cells in a dose-dependent manner. Tax-active cell lines are more susceptible to ACHP than Tax-inactive cell lines and Jurkat (IC50 values in Tax-active cell lines, Tax-inactive cell lines or Jurkat are 3.1±1.3?μM, 10.7±1.7?μM and 23.6?μM, respectively), suggesting that the growth of Tax-active cells depends on NF-κB more than Tax-inactive cells.
-
体内实验ACHP (Compound 4j) is orally bioavailable in mice and rats and demonstrates significant in vivo activity in anti-inflammatory models (arachidonic acid-induced mouse ear edema model). ACHP has reasonable aqueous solubility (0.12 mg/mL in pH 7.4 isotonic buffer) and excellent Caco-2 permeability (Papp 62.3×10-7 cm/s), and demonstrates orally bioavailability in mice (BA: 16%) and rats (BA: 60%). The favourable bioavailability of ACHP in rats is likely due to its low clearance (0.33 L/h/kg). In an acute inflammation model, ACHP exhibits oral efficacy at 1 mg/kg in a dose-dependent manner.
-
同义词IKK-2 Inhibitor VIII
-
通路Others
-
靶点Other Targets
-
受体IKKα|IKKβ
-
研究领域——
-
适应症——
化学信息
-
CAS Number406209-26-5
-
分子量400.9
-
分子式C21H25ClN4O2
-
纯度>98% (HPLC)
-
溶解度DMSO:45 mg/mL (112.25 mM; Need ultrasonic)
-
SMILESN#CC1=C(C=C(N=C1N)C2=C(C=CC=C2OCC3CC3)O)C4CCNCC4.[H]Cl
-
化学全称——
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1.Murata T, et al. Synthesis and structure-activity relationships of novel IKK-beta inhibitors. Part 3: Orally active anti-inflammatory agents. Bioorg Med Chem Lett. 2004 Aug 2;14(15):4019-22.



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn











